
Researchers at the University of Texas at Austin are utilizing imaging systems to analyze the chemical and mechanical qualities of cancer cells to determine the best treatment method. The future of cancer treatment depends on optics.
Aggressive cancers are often treated with a combination of therapeutics and while this may help to address the heterogeneity of cancer cells, it often does not effectively rid the body of them. Pancreatic cancer is one of the most invasive cancers with an estimated five-year survival rate of 10%.1 A significant factor in the malignant quality of pancreatic cancer is the inability for clinicians to accurately predict the cancer cells’ susceptibility to specific treatments. Additionally, while pancreatic cancer is usually treated aggressively, it is also difficult to understand or accurately estimate how invasive the cancer may be. Without knowing these key behavioral cell attributes it is difficult to improve survival rates in pancreatic or similarly aggressive cancers.
Researchers at the Parekh Lab understand the barriers clinicians face in cancer treatment. They are developing an optical system that combines imaging methods to gain insight into cancer cell behavior. The system is a mechano-chemical flow cytometer (MCFC) and utilizes spectroscopy and flow cytometry to analyze the biomechanical and biochemical properties of cancer cells. The technology serves to recognize and quantify cell parameters, such as surface contour or other heterogenetic qualities. This analysis will allow clinicians to gain knowledge on invasive cancer cells and form better treatment plans to combat aggressive behavior.
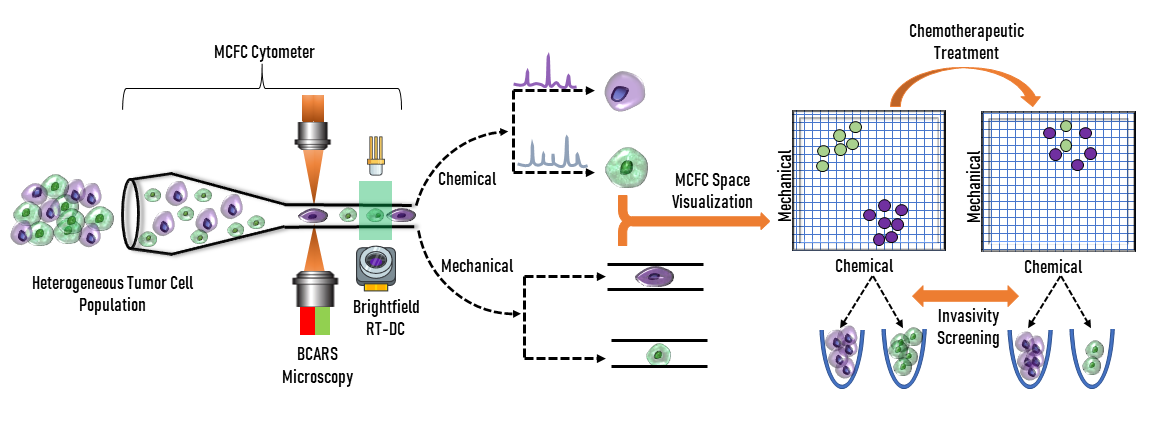
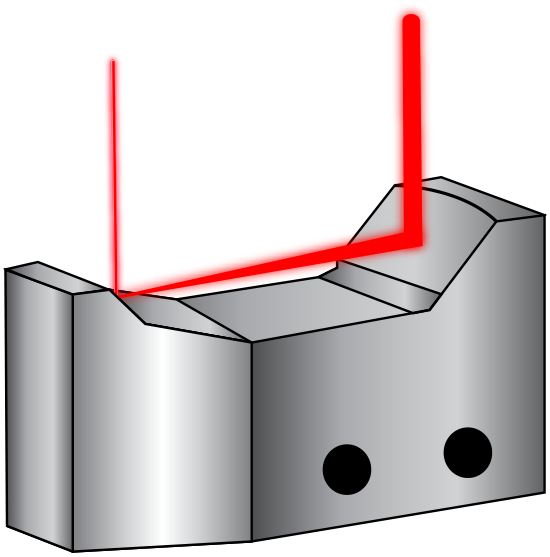
The first component of the MCFC involves spectroscopy to analyze the chemical make-up of cancerous cells. The MCFC utilizes broadband coherent anti-Stokes Raman spectroscopy (BCARS) which allows analysis to be completed without biochemical markers. In this system, a supercontinuum laser source, which uses nonlinear processes to produce an extremely broad wavelength range, is used in combination with a reflective beam expander to excite the cells. This excitation allows for label-free analysis of the chemical qualities as each individual cell has a unique vibrational signature, meaning that biochemical tagging is not required. Cell tagging can cause damage to cells, specifically to proteins within the cell, and could skew data. Protein tags can obstruct the chemistry or structure of a protein, which has the potential to change the binding affinity of the protein to other proteins with which it would normally interact. A reflective beam expander is used in BCARS because the supercontinuum source is not monochromatic. Therefore, the reflective beam expander must correct for chromatic aberration to minimize divergence differences between wavelengths. They are composed of a concave and convex mirror instead of transmissive lenses like conventional beam expanders (Figure 2). Their curved mirrors also correct for spherical aberration.
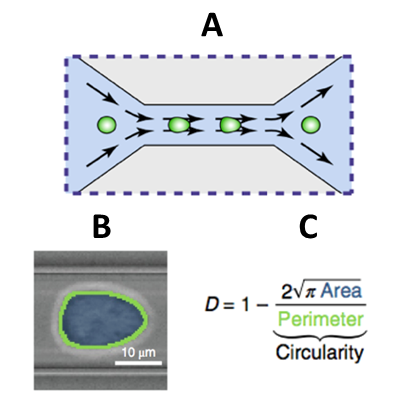
Biomechanical qualities of cancerous cells also allow for further insight into the behavior of the cancer in a patient’s body. To obtain this information, a real-time deformability cytometer (RT-DC) is used. An RT-DC works by submerging cells of interest in fluid and then sending this mixture through a small tube. The cells will be squeezed causing them to deform. How the cells behave because of constriction is what allows scientists to categorize them. Flow cytometry is another label-free method of imaging and allows cells of interest to not become damaged or compromised in the analysis process. Brightfield imaging is utilized to study surface contours and a complementary metal–oxide–semiconductor (CMOS) camera is employed to capture images for analysis. Upon imaging, cells deformities are quantified by a deformability index (DI). By comparing the DI of cells before and after chemotherapeutics or other treatments, cell groups may be identified as resistant to certain forms of therapy. These cell groups may also inform clinicians of the metastaticity of the cancer as resistance to treatment could possibly act as an indicator to potential invasive behavior, although this technique is still under development.
Looking to the future, researchers at the Parekh Lab are working to combine the technologies of BCARS and RT-DC into a commercially available MCFC. The device must first be compared to the technology that is currently widely-used in the industry: fluorescent activated cell-sorting (FACS). FACS uses fluorescent tagging to sort cells based upon the fluorescent qualities and light scattering of input cells. The new MCFC technique will allow clinicians to perform phenotypic assessments of cells in a similar fashion without the tagging to better understand cancer cell heterogeneity. Current techniques are also limited to tagging the surface of cells, which does not necessarily describe the inside of the cell. The deformation analysis of RT-DC allows it to characterize internal properties of the cells and potentially reveal more useful information.
Future implementations in clinical settings could be life-changing for cancer patients. The current studies being done by the Parekh Lab focus on pancreatic cancer cells. However, this technology could also be implemented in several other cancer treatment measures. Once commercially available, healthcare providers will be able to analyze a patient’s cancer cells label-free and gain a better understanding of how the cancer itself will likely react to different types of treatment. Post-therapy analysis will then give direction to clinicians on how they should proceed and give a better idea of the aggressive nature of the cancer. This knowledge is imperative to providing life-saving treatment to patients and has the potential to increase long-term survival rates.

The Edmund Optics Educational Award Program serves to encourage the continuation of innovative research in optics at non-profit colleges and universities. The Parekh Lab at the University of Texas at Austin was awarded the 2020 Silver Educational Award for their work in constructing a mechano-chemical flow cytometer to improve cancer treatment based on cellular analysis. The award granted $7,500 worth of products to the Parekh Lab and has allowed them to purchase quality objectives and reflective beam expanders. The eventual construction of the MCFC will improve the effectiveness of cancer treatment plans formulated by physicians. This could result in increased survival rates in fatal cancers and allow treatment to become a more data-driven practice. Edmund Optics was honored to provide the 2020 Silver Education Award to the Parekh Lab for their work towards the actualization of MCFC in clinical practices to help fight cancer.












or view regional numbers
QUOTE TOOL
enter stock numbers to begin
Copyright 2024, Edmund Optics Singapore Pte. Ltd, 18 Woodlands Loop #04-00, Singapore 738100
California Consumer Privacy Acts (CCPA): Do Not Sell or Share My Personal Information
California Transparency in Supply Chains Act